Help us create an Environmental Health APPG
Join our campaign by urging your local MP to support the formation of an All-Party Parliamentary Group (APPG) on environmental health.
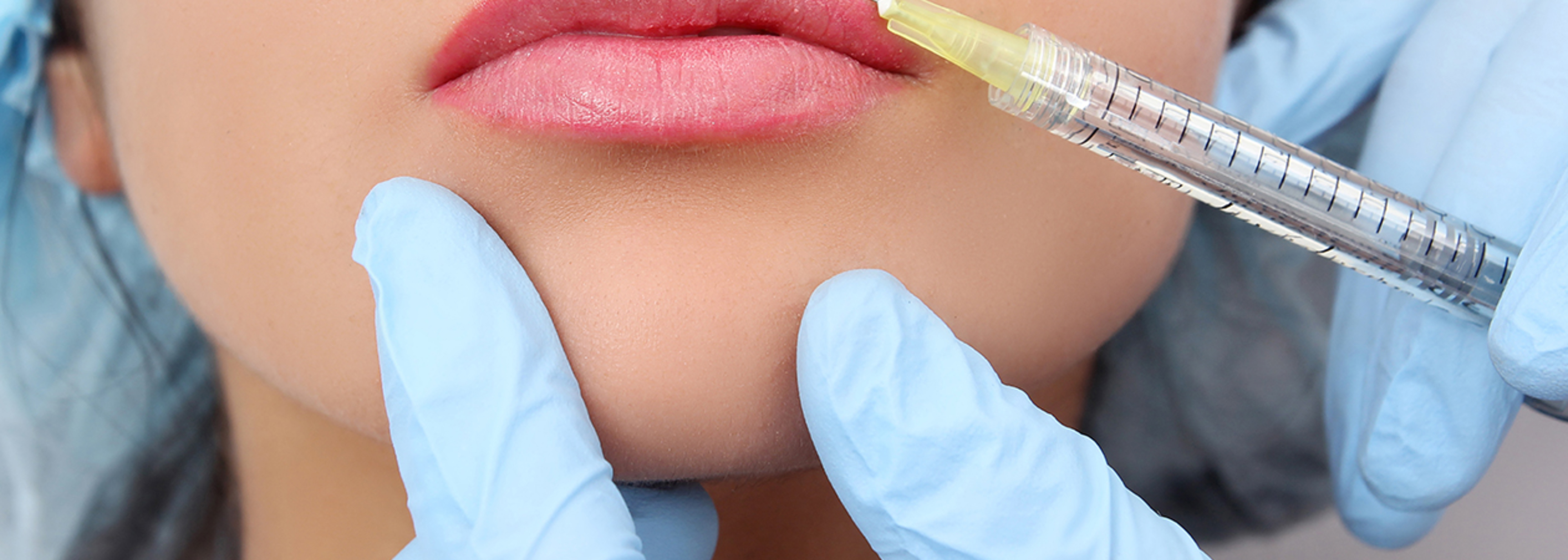
Surgeons voice concern about the safety of fillers provided by the high street beauty industry.

Help us create an Environmental Health APPG
Join our campaign by urging your local MP to support the formation of an All-Party Parliamentary Group (APPG) on environmental health.